Phenotypic plasticity is the ability of an organism to change its morphology, physiology, or behavior in response to environmental change.
Bulgy tadpoles: a unique morphological defenses
A common form of phenotypic plasticity is an Inducible defense, which typically involves an individual’s altering its behavior, life history or morphology in response to predation risk. Rana pirica frogs tadpoles acquire a unique bulgy body when they develop in close proximity to predatory larvae of Hynobius retardatus salamanders (Kishida & Nishimura 2004). Expression of the bulgy phenotype by R. pirica tadpoles effectively prevents them from being swallowed by the larvae, which are gape-limited predators.
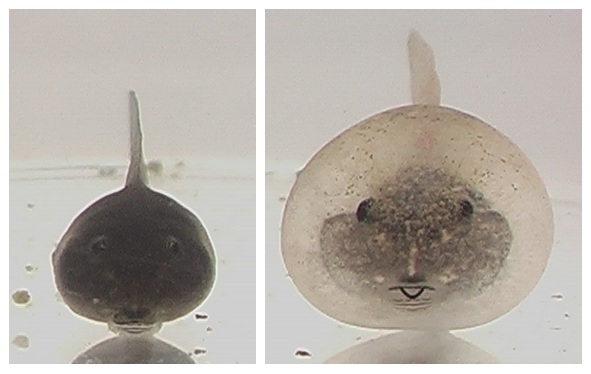
A Tadpole with the non-defensive phenotype (left), and one with the defensive bulgy phenotype (Right)
Reference: Kishida, O. & Nishimura, K. (2004) Bulgy tadpoles: inducible defense morph. Oecologia 141:414-421.
Predator-specific inducible morphological defenses.
Prey organisms need to use different defensive tactics against predators that employ different hunting methods. We think that Rana pirica tadpoles use phenotypic plasticity in just this way, because they can not only acquire a bulgy phenotype as a defense against the larval salamanders, they can also acquire a “high-tail” phenotype in response to predation risk from dragonfly (Aeshna nigroflava) larvae, which bite their prey instead of swallowing it whole. We interpret these predator-specific phenotypes as predator-specific defenses (Kishida & Nishimura 2005).
Expression of these predator-specific defensive phenotypes by the tadpoles is highly flexible (Kishida & Nishimura 2006). Even a tadpole that has already expressed one predator-specific phenotype can modify its morphology if the predator is removed or exchanged for the other predator species. When the predator species is removed, a tadpole with either predator-specific phenotype reverts its phenotype to the non-defensive one. If one predator species is exchanged for the other, then the tadpole changes its phenotype to the one specific for the new predator. Thus, a tadpole can flexibly shift its phenotype from the bulgy morph to the high-tail morph, or vice versa, depending on whether it must defend itself against salamander or dragonfly larvae (Kishida & Nishimura 2006).

Three tadpole morphs: (top) Non-defensive, (middle) dragonfly-induced high-tail, and (bottom) salamander-induced bulgy morphs
References:
- Kishida, O. & Nishimura, K. (2005) Multiple inducible defences against multiple predators in the anuran tadpole, Rana pirica. Evolutionary Ecology Research 7:619-631
- Kishida, O. & Nishimura, K. (2006) Flexible architecture of inducible morphological defenses. Journal of Animal Ecology 75:705-712.
Antagonistic phenotypic plasticity

Salamander larvae with displaying the offensive (above) and the non-offensive phenotype (bottom)
Just as R. pirica frog tadpoles can acquire a defensive phenotype to protect themselves from predation, H. retardatus salamander larvae can exhibit an offensive phenotype that makes them more effective predators. When salamander hatchlings are raised among R. pirica tadpoles or conspecifi hatchlings, some of them acquire an enlarged gape during their early development (Michimae & Wakahara 2002). They are especially likely to enlarge their gape in the presence of small tadpoles (Michimae & Wakahara 2002). This broadened gape, which allows the salamander larvae to swallow large prey items more easily, is a good example of ‘inducible offense’ (Takatsu & Kishida 2013). Interestingly, Rana pirica tadpoles that are exposed to predation pressure from salamander larvae with this offensive morph develop an even bulgier phenotype (relative to body length), compared with tadpoles exposed to predation pressure from salamander larvae with the non-offensive phenotype (Kishida et al. 2006). This variable response of the tadpoles to this gape-limited predator may be adaptive, because its hard for salamander larvae that don’t have the offensive phenotype to swallow the bulgy tadpoles (Takatsu & Kishida 2013). In fact, R. pirica tadpoles may have evolved the inducible bulgy defense morph as a response to the variable expression of the offensive morph by salamander larvae.
References:
- Michimae H. & Wakahara M. (2002) A tadpole-induced polyphenism in the salamander Hynobius retardatus. Evolution 56:2029-2038.
- Kishida O., Mizuta Y. & Nishimura K. (2006) Reciprocal phenotypic plasticity in a predator-prey interaction between larval amphibians. Ecology 87:1599-1604.
- Takatsu K. & Kishida O. (2013) An offensive predator phenotype selects for an amplified defensive phenotype in its prey. Evolutionary Ecology 27:1-11.
Geographic variation of inducible defenses and its genetic basis
To deepen our understanding of the evolution of inducible defenses, we examined genetic variation and geographic differentiation in the inducible bulgy defense of R. pirica tadpoles (Kishida et al. 2007). We crossed frogs from a mainland population where there were predaceous salamanders with other mainland frogs or with frogs from a population from an island without predaceous salamanders, and we also crossed frogs from the island population with other island frogs. Then we raised the resulting offspring in the presence or absence of H. retardatus salamander larvae. We found that tadpole offspring of mainland frogs were better able to express the inducible morphology (i.e., they acquired a more bulgy body) than the offspring of frogs from the predator-free island. In addition, expression of the bulgy morph by mainland-island hybrids and expression of the bulgy morph in mainland–island hybrids was intermediate between the phenotypes produced by the pure crosses. In this experiment, the expression of the bulgy morph was the same whether the mother or the father came from the mainland population. These experimental results support our hypothesis that geographic variation in the inducible defenses of R. pirica reflects the additive effects of autosomal alleles that differ depending on a population’s historical exposure to H. retardatus salamanders.

Left: Mainland x mainland tadpole not exposed to predation. Middle: Island x Island tadpole exposed to predation risk for three weeks. Right: Mainland x mainland tadpole exposed to predation risk for three weeks.
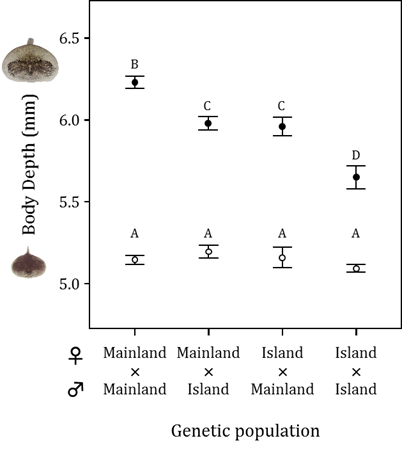
Size-adjusted body depth of Rana pirica tadpoles produced by four different genetic crosses and exposed (solid circles) or not exposed (control; oplen circles) to predation by larval salamanders. A larger body depth means a more bulgy morph. Significantly different means are labeled with different letters (from Kishida et al. 2007).
Reference: Kishida O., Trussell GC. & Nishimura K. (2007) Geographic variation in a predator-induced defense and its genetic basis. Ecology 88:1948-1954
Phenotypic plasticity of multiple traits
An adaptive phenotypic response to predation can impose a new challenge on a prey species. How do organisms cope with the new challenge? The dragonfly, A. nigroflava, preys on H. retardatus salamander larvae as well as on frog tadpoles. These larvae sometimes exhibit a surfacing behavior in order to take in oxygen from the air, but they surface less frequently in the presence of a dragonfly predation risk, because surfacing makes them conspicuous to this visual predator. To compensate for the unavoidable cost (hypoxia) associated with this induced behavioral defense, the salamander larvae develop enlarged gills (Iwami et al. 2007). Interestingly, gill enlargement is induced in response to not only hypoxic conditions but also chemical cues from the dragonfly. This finding suggests that organisms may evolve functional interactions among multiple inducible traits that allow them to cope with the variable nature of the selective environment.
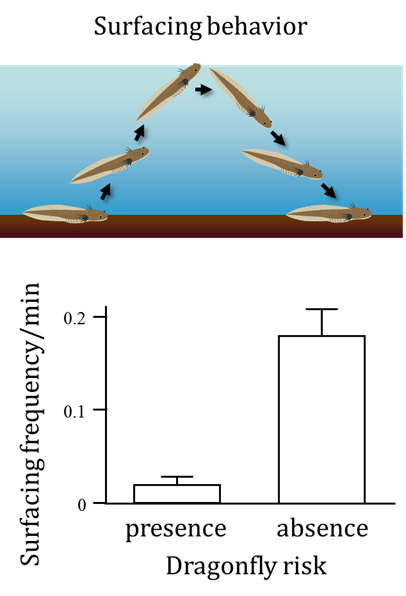
Surfacing frequency of Hynobius retardatus salamander larvae in the presence (left) and absence (right) of a dragonfly predation risk (from Iwami et al. 2007).

The salamander larva on the left was not exposed to predation, whereas the one on the right was exposed to a predation risk from dragonflies.
Reference: Iwami T., Kishida O. & Nishimura K. (2007) Direct and indirect induction of a compensatory phenotype that alleviates the costs of an inducible defense. PLoS one 2:e1084.
Surviving in a cannibalistic environment: growth and developmental acceleration of pre-feeding salamander hatchlings
In many fish and amphibian species, vast numbers of embryos may hatch at the same time. This may cause the hatchlings to be exposed to intensive cannibalism by conspecifics (members of the same species). How do hatchlings survive this vulnerable early life stage?
The ability of an H. retardatus salamander larva to successfully cannibalize its conspecifics is highly dependent on its gape width (how wide it can open its mouth) relative to the head width of its conspecifics. Similarly, its ability to escape cannibalism depends on its head being too wide for it to be easy prey for conspecifics. This means that the faster a hatchling grows before it begins to feed, the more likely it is to survive later conspecific interactions. To test this hypothesis, we reared salamander hatchlings alone or with conspecifics. Those reared with conspecifics became larger and developed faster than those reared alone, they started feeding sooner, and they could swim faster. This is experimental evidence of adaptive acceleration of growth and development of hatchlings in the pre-feeding stage. Next, we performed predation trials to demonstrate the advantages of early growth and developmental acceleration in cannibalistic interactions. Compared with the hatchlings reared alone, the hatchlings reared with conspecifics were more successful at cannibalizing smaller hatchlings and were also much less likely to be cannibalized themselves by larger conspecifics. Because salamander larvae that cannibalize other larvae in their early developmental period exhibit rapid growth and metamorphose early and at a larger size than non-cannibalistic individuals, growth and developmental acceleration are likely key mechanisms for life history success.



